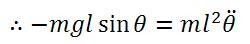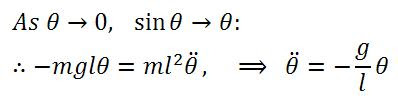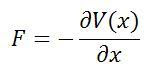There's a hidden birthday message, can you find it?
So
here's the c
ase; as is the case with the USA, Singa
porean soldiers are not allowed to take illegal drugs (besides the usual smoke I guess, which is
perfectly legal). The question that I pose to you now, is:
If you'd like to do a survey to find out how many Singaporean soldiers consume illegal drugs, how would you do it?
You must keep in mind that if they do confess or are caught answering "Yes", then they will face the death penalty or some other kind of huge fine. The nice guy you are, you'd want to come up with a way to prevent this, and yet come up with some sort of estimate of figures.
Knowing this blog, it's definitely a statistical trick that you'll have to employ, but what? Heh.-------------------------------------------
Well, the thing is, you've got to let them confess in secret, but yet you have to know what they confessed somehow. But you need to ensure their anonymity and safety as well!
As such, leaving any high-technology mind reading
devices out, we
are left with no choice but to play Jedi mind tricks.
Yeah right. We just use what we learnt in high school - that's right, simple statistics, for a good estimation.
We come up with three t
ypes of cards, as shown (of course their backs will be all of the same colour):

Each type of card is associated with one type of questio
n - these quest
io
ns need not be written on the card, but may be posed to the soldiers verbally. Now, to ensure that the soldiers draw out a card at random, so the chance of drawing a red, blue or green card is 1/3 for all, we have say, a
good number of cards (say 72 cards) laid down like this:

So now, the chance or probability that any soldier draws the green, blue or red card, is exactly 1/3, even if he or she has a propensity or tendency to draw from the corners or from the middle of the deck. Make sure that the cards are laid in the order as shown for the probability to be true.
Let's give it a go; let's say for example, that out of 12000 soldiers who were surveyed, 5600 of them answered "Yes". Assuming all soldiers are sane, and that they completely understand English and are well, disciplined enough to not want to play with the system, they'll be truthful.
Therefore, all "Yes" replies must mean that the soldiers either chose the red or blue card.
Since 12000 is a huge enough number, and this is a fair test, we should be confident enough to say that on average, we expect 4000 soldiers to say "Yes" to the question of whether "Is this card red?"
If that is the case, then we expect that on average, at least 1600 soldiers do take some kind of illegal drug.
On the other hand, we also expect 4000 soldiers to say "Yes" to the question of "Do you take drugs?"
So what does all these mean? It means that
on average, we expect at best, 1600 soldiers to be taking drugs, but at worst, 4000 soldiers to be taking drugs.
It'd be good to perform another test on another group first, to obtain the standard deviation
! Haha.
So... what do you think? Aren't statisticians rather useful? Heh.